Basic Concepts
Introduction
Pacemakers and implantable cardioverter defibrillators (ICDs) have been in use for more than 20 years. With expanding indications and an ever-growing elder population, emergency physicians must be familiar with emergent indications for their application, discontinuation, and complications arising from a patients’ existing device. This article introduces the common problems encountered with pacemakers and ICDs, and rescue techniques that may aid in treating such complications.
Pacemaker and ICD basics
Permanent pacemakers are implanted devices that provide electrical stimuli, thereby causing cardiac contraction when intrinsic myocardial electrical activity is inappropriately slow or absent. These devices sense intrinsic cardiac electric potentials and, if too infrequent or absent, they transmit impulses to the heart to stimulate myocardial contraction.
An ICD is a specialized device designed to directly treat a cardiac tachydysrhythmias. If a patient has a ventricular ICD and the device senses a ventricular rate that exceeds the programmed cut threshold, the device performs antitachycardia pacing. With antitachycardia pacing, the device fires a preset number of rapid pulses in succession in an attempt to terminate the ventricular tachycardia. If unsuccessful, the device will perform a cardioversion/defibrillation.
Newer-generation ICDs are also equipped with an intrinsic bradycardia demand pacing system, and some, if required, are a combination of an ICD and a pacemaker. It is important to be aware that some of the older models (more than 10 years old) may lack this function.
Pacemaker and ICD anatomy
Pacing systems consist of a pulse generator and pacing leads. With permanent systems, endocardial leads are inserted transvenously and advanced to the right ventricle and/or atrium where they are implanted into the myocardial tissue. The pulse generator is placed subcutaneously or submuscularly in the chest wall.
Pulse generators contain a battery as well as sensing, timing and output circuits. The battery (most commonly lithium-iodide) typically has a life span of 5-10 years.
During pacemaker placement, signal amplitude and width are set high enough to reliably achieve myocardial capture, yet low enough to maximize battery life.
Temporary systems use an external pulse generator with leads placed either transcutaneously or transvenously. Transcutaneous leads are the easiest and most convenient to use for rapid application of temporary pacing and is the method of choice during ED resuscitation. Once the patient is stabilized or central venous access is gained, transvenous leads provide the most reliable pacing mechanism and are a good transition to permanent systems.
For transvenous temporary pacing, semirigid catheters are inserted through a central venous access. ECG monitoring (specifically V1) is used to track catheter positioning. For example, P-wave morphology is initially inverted and becomes upright as the catheter is in line with the SA node. QRS morphology is also initially inverted, transitioning to isoelectric and then upright as the tip is placed in the apex. An injury pattern resembling ST elevation ensures that the catheter tip is in proper positioning for pacing. Semifloating or flexible balloon-tipped catheters can be used in emergencies since they can be positioned without such monitoring.
Transcutaneous pacing is discussed in detail in a separate article (see External Pacemakers).
Pulse generators can be set to fixed-rate (asynchronous) or demand (synchronous) modes. In the asynchronous mode, impulses are produced at a set rate independent of intrinsic cardiac activity. This mode carries a small but inherent danger of producing lethal dysrhythmias should the impulse coincide with the vulnerable period of the T wave. In the synchronous mode, the sensing circuit searches for an intrinsic depolarization potential. If this is absent, a pacing response is generated. This mode closely mimics intrinsic myocardial electric activity.
Pacing Codes
The Heart Rhythm Society and the British Pacing and Electrophysiology Group (BPEG) have developed a code to describe various pacing modes.
Table 1.Pacemaker Code Used to Describe Various Pacing Modes
Table
| 1st Letter | 2nd Letter | 3rd Letter | 4th Letter | 5th Letter |
Chamber
Paced | Chamber
Sensed | Response to
Sensing | Programmability and Rate Modulation | Antitachyarrhythmia Function |
| A | A | T | P | P (pacing) |
| V | V | I | M | S (shock) |
| D | D | D | C | D (dual: pacing + shock) |
| | O | O | R | |
| | | | O | |
| 1st Letter | 2nd Letter | 3rd Letter | 4th Letter | 5th Letter |
Chamber
Paced | Chamber
Sensed | Response to
Sensing | Programmability and Rate Modulation | Antitachyarrhythmia Function |
| A | A | T | P | P (pacing) |
| V | V | I | M | S (shock) |
| D | D | D | C | D (dual: pacing + shock) |
| | O | O | R | |
| | | | O | |
Abbreviations: A, atrium; V, ventricle; D, dual (both chambers); O, none; T, triggered; I, inhibited; D, double (atrial triggered and ventricular inhibited); P, simple programmability; M, multiprogrammable; C, communicating (telemetry); R, rate adaptive.
Pacing code explanation:
A typical pacing code consists of 3-5 letters.
- The first letter indicates the chamber(s) paced.
- A: Atrial pacing
- V: Ventricular pacing
- D: Dual-chamber (atrial and ventricular) pacing
- The second letter indicates the chamber in which electrical activity is sensed.
- A, V, or D
- O is used when pacemaker discharge is not dependent on sensing electrical activity.
- The third letter refers to the response to a sensed electric signal.
- T: Triggering of pacing function
- I: Inhibition of pacing function
- D: Dual response (ie, any spontaneous atrial andventricular activity will inhibit atrial and ventricular pacing and lone atrial activity will trigger a paced ventricular response)
- O: No response to an underlying electric signal (usually related to the absence of associated sensing function)
- The fourth letter represents programmability and rate modulation.
- P: Simple programmable
- M: Multiprogrammability
- C: Communication
- R: Rate-response ("physiologic") pacing
- O: No programmability or rate modulation
- The fifth letter represents presence of antitachyarrhythmia function.
- P: Pacing (antitachyarrhythmia)
- S: Shock
- D: Dual (pacing + shock)
Although the first 3 letters are used most commonly, a 5 position code is currently in use. The first position denotes the chamber(s) paced; the second position denotes the chamber(s) sensed; the third position denotes the action(s) performed; the fourth position denotes rate response; finally, the fifth position denotes antitachyarrhythmia function.
More modern pacemakers have multiple functions. The simplest settings are VVI and AAT. The VVI mode senses and paces the ventricle and is inhibited by a sensed ventricular event. Alternatively, the AAT mode senses and paces in the atrium, and each sensed event triggers the generator to fire within the P wave.
The most common setting, DDD mode denotes that both chambers are capable of being sensed and paced. This requires two functioning leads, one in the atrium and the other in the ventricle. In the ECG, each QRS is preceded by 2 spikes, The first indicating the atrial depolarization and the second indicating the initiation of the QRS complex. Given that one of the leads is in the right ventricle, a left bundle-branch pattern may be evident on ECG. Note that a 2-wired system does not necessarily need to be in DDD mode, since the atrial or ventricular leads can be programmed off. Additionally, single tripolar lead systems are available that can sense atrial impulses and either sense or pace the ventricle. Thus, this system provides for atrial tracking without the capability for atrial pacing and can be used in patients with atrioventricular block and normal sinus node function.
Pacemaker programming can be performed noninvasively by an electrophysiology technician or cardiologist. Because of the myriad of pacemaker types, patients should carry a card with them providing information about their particular model. This information is crucial when communicating with the cardiologist about a pacer problem. However, most pacemaker generators have an x-ray code that can be seen on a standard chest radiograph. The markings, along with the shape of the generator, may assist with deciphering the manufacturer of the generator and pacemaker battery.
For further information or locations of technicians for pacemaker devices, the device company can be contacted at the 24-hour help line telephone numbers below.1
- Guidant (Boston Scientific) - 800-CARDIAC (800-227-3422)
- Medtronic - 800-MEDTRONIC (800-633-8766)
- St. Jude Medical - 800-722-3774
Pacemaker and ICD Indications
Pacemaker indications
Absolute indications for pacemaker placement include the following:
- Sick sinus syndrome
- Symptomatic sinus bradycardia
- Tachy-brady syndrome
- Atrial fibrillation with sinus node dysfunction
- Complete atrioventricular block (third-degree block)
- Chronotropic incompetence (inability to increase the heart rate to match a level of exercise)
- Prolonged QT syndrome
- Cardiac resynchronization therapy with biventricular pacing
Relative indications include the following:
- Cardiomyopathy (hypertrophic or dilated)
- Severe refractory neurocardiogenic syncope
Temporary emergency pacing is indicated for therapy of significant and hemodynamically unstable bradydysrhythmias and for prevention of bradycardia-dependent malignant dysrhythmias. Examples include refractory symptomatic sinus node dysfunction, complete heart block, alternating bundle-branch block, new bi-fascicular block, and bradycardia-dependent ventricular tachycardia. Examples of indications for prophylactic temporary pacing include insertion of a pulmonary artery catheter in a patient with an underlying left bundle-branch block, use of medications that may cause or exacerbate hemodynamically significant bradycardia, prophylaxis during the perioperative period surrounding cardiac valvular surgery, Lyme disease or other infections (Chagas disease) that cause interval changes, and prolonged PR intervals.
ICD indications2
(For further reading and a detailed list of indications, see ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities.3 )
Indications for ICDs include the following:
- Survivors of cardiac arrest due to ventricular fibrillation (VF) or hemodynamically unstable sustained ventricular tachycardia (VT) after evaluation to define the cause of the event and to exclude any completely reversible causes
- Structural heart disease and spontaneous sustained VT (stable or unstable)
- Syncope of undetermined origin with clinically relevant, hemodynamically significant sustained VT or VF induced at electrophysiological study
- Patients with left ventricular ejection fraction (LVEF) <35%>
- Nonischemic dilated cardiomyopathy with LVEF ≤35% and NYHA functional Class II or III
- Left ventricular (LV) dysfunction due to prior MI, ≥40 days post-MI, with LVEF <30%,>
- Nonsustained VT due to prior MI, LVEF <40%,>
- Unexplained syncope, significant LV dysfunction, and nonischemic dilated cardiomyopathy
- Sustained VT and normal or near-normal ventricular function
- Hypertrophic cardiomyopathy who have 1 or more major risk factors for sudden cardiac death (SCD)
Initially, ICDs were used for secondary prevention in patients who had documented life-threatening ventricular arrhythmias and survivors of cardiac arrest. A meta-analysis of 3 large trials, principally, Antiarrhythmics vs Implantable Defibrillator (AVID) study,4 the Cardiac Arrest Study Hamburg (CASH),5 and the Canadian Implantable Defibrillator Study (CIDS),6 showed patients in the ICD group had significant reduction in all-cause death and death from arrhythmia. Further analysis of the CIDS trial with an 11-year follow-up revealed that the benefit of ICD over amiodarone increased with time.7
Recent trials suggest ICDs are beneficial for primary prevention of sudden cardiac death. Multiple trials have demonstrated that primary prevention in post-MI patients with reduced ejection fraction, nonsustained VT, and inducible nonsuppressible VT in electrophysiological testing with ICD over conventional medical therapy saved lives.8,9 Further studies have shown that primary prevention using ICDs in other patient subset groups is also beneficial.10,11,12,13,14
For further detailed discussion and evidence supporting ICDs, see Implantable Cardioverter-Defibrillators.
Magnet Inhibition
Placing a magnet over a permanent pacemaker closes an internal reed switch to inhibit sensing. This temporarily "reprograms" the pacer into asynchronous mode. It does not turn the pacemaker off. Each pacemaker type has a unique asynchronous rate for beginning-of-life (BOL), elective replacement indicator (ERI), and end-of-life (EOL). Therefore, application of a magnet can determine if the pacer's battery needs to be replaced. Further interrogation or manipulating of the device should be performed by an individual skilled in the technique.
Although many different branded pacemaker/ICD magnets are available, emergency physicians should be aware that in general any pacemaker/ICD magnet can be used to inhibit the device.
It is worth mentioning that, when a magnet is applied to an ICD, it can temporarily turn off defibrillation therapy without altering its backup bradycardia pacing, this is further described later in the article .
Pacemaker Malfunctions and Complications
Major pacemaker malfunctions include the following:
- Failure to output
- Failure to capture
- Failure to sense
- Pacemaker-mediated tachycardia
- Runaway pacemaker
- Pacemaker syndrome
- Twiddler's syndrome
Failure to output
Failure to output occurs when no pacing spike is present despite an indication to pace. This may be due to battery failure, lead fracture, fractured lead insulation, oversensing (inhibiting pacer output), poor lead connection at the takeoff from the pacer, and "cross-talk" (ie, a phenomenon occurring when atrial output is sensed by a ventricular lead in a dual-chamber pacer).
Management of pacer output complications includes medications to increase the intrinsic heart rate and placement of a temporary pacer. A chest radiograph is warranted to check pacer leads and to evaluate for possible lead fracture, which occurs most commonly at the clavicle/first rib. The patient's pacer identification card should be obtained and his/her electrophysiologist/cardiologist consulted.
Failure to capture
Failure to capture occurs when a pacing spike is not followed by an atrial or a ventricular complex. This may be due to lead fracture, lead dislodgement, fractured lead insulation, an elevated pacing threshold, myocardial infarction at the lead tip, drugs (eg, flecainide), metabolic abnormalities (eg, hyperkalemia, acidosis, alkalosis), cardiac perforation, poor lead connection at the takeoff from the generator, and improper amplitude or pulse-width settings. Fibrosis at the endocardial surface where leads were implanted may also occur in the weeks following pacemaker implantation. The fibrosis may create an electrical resistance barrier preventing ventricular depolarization.
Managing pacer capture complications is similar to treating output complications, with extra consideration given to treating metabolic abnormalities and potential myocardial infarction. Temporary pacing is used to stabilize the patient until an electrophysiology technician or cardiologist can further evaluate the pacemaker.
Oversensing
Oversensing occurs when a pacer incorrectly senses noncardiac electrical activity and is inhibited from pacing. This may result in a heart rate lower than the preset rate. This form of output failure may be due to muscular activity (particularly the diaphragm or pectoralis muscles), electromagnetic interference (MRIs), or fractured lead insulation. Oversensing is one condition that is diagnosable and treatable with magnet application. As mentioned before, magnet application will convert the pacemaker to asynchronous mode, and it will then operate at the preset rate.
Of note, recently, it has been reported that cellular phones held within 10 cm of the pulse generator may elicit this response.
Individual ICD manufacturers also have recommendations for unsafe devices that may interact with the ICD. (For example,
Safe and Unsafe devices - Medtronic Brochure for Patients15 )
Undersensing
Undersensing occurs when a pacer incorrectly misses intrinsic depolarization and paces despite intrinsic activity. The pacemaker is more or less operating in asynchronous mode. This may be due to poor lead positioning, lead dislodgment, magnet application, low battery, or myocardial infarction. Management is similar to that for other types of failures.
Pacemaker-mediated tachycardia
A premature ventricular contraction (PVC) in a dual-chamber pacemaker may precipitate a
pacemaker-mediated tachycardia. If a premature ventricular contraction (PVC) is transmitted in a retrograde manner through the AV node, it may, in turn, depolarize the atria. This atrial depolarization is detected by the atrial sensor, which then stimulates the ventricular leads to fire, hence creating an endless loop. Although the maximum rate is limited by the pacemaker’s programmed upper limit, the possibility of developing ischemia exists in susceptible patients. This is another opportunity to use a magnet to diagnose and treat the arrhythmia. The magnet will place the pacemaker into asynchronous mode and sensing will be deactivated, thus preventing continuation of the reentrant dysrhythmia.
Runaway pacemaker
A malfunction of the pacemaker generator resulting in a life-threatening rapid tachycardia (up to 400 bpm) is known as runaway pacemaker. The generator may malfunction from various causes, although most commonly it is a battery failure or external damage. This rare medical emergency requires immediate action. An external magnet may induce slower pacing, but it is possible that the device will not respond to magnet application and more aggressive measures may be necessary. If a patient becomes unstable, treatment involves making an incision in the chest wall over the pacemaker and severing the pacemaker leads from the generator. Note that the patient may require temporary packing as a result.
Pacemaker syndrome
Pacemaker syndrome is a phenomenon where a patient feels symptomatically worse after pacemaker placement and presents with progressively worsening symptoms of congestive heart failure (CHF). This is mainly due to the loss of atrioventricular synchrony whereby the pathway is reversed and now has a ventricular origin. The atrial contribution to the preload is lost and cardiac output as well as blood pressure fall. Immediate treatment is mainly supportive, whereas long-term treatment involves altering the pacemaker to restore atrial-ventricular synchrony. For example, this may require changing the pacemaker from single-chamber to dual-chamber pacing.
For further reading, see
Pacemaker Syndrome.
Twiddler's syndrome
Some patients will persistently disturb and manipulate the pacemaker generator resulting in malfunction. A chest radiograph may reveal twisting or coiling, or lead fracture, dislodgement, or migration. This situation will require surgical correction with further patient education and counseling.
Pacemaker complications
Pacemaker complications include malfunction due to mechanical factors such as pneumothorax, pericarditis, infection, skin erosion, hematoma, lead dislodgment, and venous thrombosis (also see
Pacemaker Malfunction). Treatment depends on the etiology. Pneumothoraces may require medical observation, needle aspiration, or even chest tube placement. Erosion of the pacer through the skin, while rare, requires device replacement and systemic antibiotics. Hematomas may be treated with direct pressure and observation, rarely requiring surgical drainage. Lead dislodgment generally occurs within 2 days of device implantation pacer and may be seen on chest radiography. Free-floating ventricular leads may trigger malignant arrhythmias. Device-associated venous thrombosis is rare, but generally presents as unilateral arm edema. Treatment includes extremity elevation and anticoagulation.
Advanced life support protocols, including defibrillation may safely be performed for patients with pacemakers in place. Sternal paddles are placed at a safe distance (10 cm) from the pulse generator. Temporary pacing may become necessary in cases of myocardial infarction, as the current pacemaker discharge settings may be insufficient to stimulate ventricular contraction.
ICD Complications
Major implantable cardioverter defibrillator (ICD) complications are similar to those found in pacemakers and include operative failures, sensing and/or pacing failures, inappropriate cardioversion, ineffective cardioversion/defibrillation, and device deactivation.
Operative failures are identical to those found in regular pacemakers.
ICD sensing problems similar to those seen with pacers may also occur. An example of appropriate failure to treat is when a device has a cut-off rate of 180 bpm. If ventricular tachycardia occurs at 160 bpm, the device, appropriately, fails to cardiovert the patient since the rate of the dysrhythmia is below the programmed threshold.
Inappropriate cardioversion is the most frequent ICD-associated complication. This should be considered when a patient presents in atrial fibrillation or reports multiple shocks in rapid succession without preceding symptoms. Other causes include T wave oversensing, lead fracture, lead insulation breakage, electrocautery, MRI, and electromagnetic interference.
Magnet use inhibits further ICD discharge. It does not, however, inhibit bradycardiac pacing. In some devices, application of a magnet produces a soft beep for each QRS complex. If the magnet is left on for approximately 30 seconds, the ICD is disabled and a continuous tone is generated. To reactivate the device, the magnet must be lifted off the area of the generator and then replaced. After 30 seconds, the beep returns for every QRS complex.
- Indications for ICD deactivation
- End-of-life care (after a discussion with the patient and family)
- Inappropriate shocks
- During resuscitation
- With transcutaneous pacing (external pacing can cause an ICD to fire)
- During procedures such as central lines or surgery with electrocautery
Failure to deliver a shock may be caused by failure to sense, lead fracture, electromagnetic interference, and inadvertent ICD deactivation. Management includes external defibrillation or cardioversion and antidysrhythmic medications.
Ineffective cardioversion may result from inadequate energy output, rise in defibrillation threshold (possibly due to antiarrhythmic medications such as amiodarone, flecainide, phenytoin), myocardial infarction at the lead site, lead fracture, insulation breakage, and dislodgment of the leads or cardioversion patches. The latter is occasionally still seen in patients with ICDs implanted during open chest surgery prior to 1993.
Many ICDs deliver a programmed set of therapies per dysrhythmic episode. The number of therapies per episode is manufacturer specific. If a delivered therapy does not terminate the arrhythmia, the device proceeds to the next programmed therapy. For example, a total of 6 attempts at defibrillation are attempted per episode of ventricular fibrillation. The device attempts defibrillation and then reevaluates the cardiac rhythm. If the arrhythmia persists, it delivers therapy number two and so on, until all 6 attempts have been delivered. Once this occurs, the device does not deliver therapy until a new episode is declared. Note that as mentioned earlier in this article, initial therapy for ventricular tachycardia may be anti-tachycardia pacing (also known as overdrive pacing) rather than simple cardioversion.
ICDs do not prevent all sudden deaths, and acknowledging that cardiac arrest is not necessarily an ICD malfunction is important. The device may have properly delivered the required shocks for the triggering rhythm but was ineffective in resolving it.
Resuscitation
If a patient enters a life-threatening cardiac arrhythmia, advanced cardiac life support (ACLS) protocols should be initiated immediately. Although an implantable cardiac defibrillator (ICD) will attempt defibrillation, chest compressions should be continued. Note that some of the current may enter the rescuer, and, besides some mild discomfort, there has never been a reported case of rescuer injury from this.1 Ventricular tachycardia and ventricular fibrillation refractory to ICD defibrillation will require external defibrillation and/or antiarrhythmic medications as dictated by ACLS protocols. If external defibrillation is required, attempt to keep the generator at least 10 cm away and out of the shock wave. Defibrillation that affects the generator may cause total device failure. However, do not withhold therapy for fear of damaging the ICD.
If rescuers are uncomfortable with ICD discharge during resuscitations, it is indicated to deactivate the ICD with a magnet, as described in Magnet Inhibition.
Central venous catheters
Pacemaker or ICD leads placed in the venous system often have surrounding thrombosis with 20% of patients having complete occlusion at 2 years.16 If the metal guidewire contacts the lead system during central line placement, there may be enough noisy artifact to trigger an inappropriate shock. Consideration should be given to either avoid a metal guidewire or deactivate the ICD during central line placement. Although the contralateral subclavian or internal jugular vein can be cannulated with care, the femoral vein access is a much safer option.
Admission and Difficulties Surrounding a Safe Discharge
One of the most difficult decisions after a patient presents to the ED complaining of an ICD discharge is to determine if the discharge was appropriate. Whenever possible, the device should be investigated. Unless the shock and rhythm that preceded it was witnessed, it is not possible to determine shock appropriateness without investigation. Reasons for admission may include the following: device investigation to determine whether there is an eminent battery failure (multiple shocks will deplete battery life); addition of antiarrhythmic medications; treatment of myocardial infarction, which may be linked to the initial discharge; treatment of patient discomfort; and to give psychological support (up to 35% of people develop anxiety disorder following ICD placement).17
Summary
The goal of this article is to orient the reader to the basic function and use of pacemakers/ICDs and important complications of such devices, thus allowing the ED clinician to better understand and troubleshoot the causes of pacemaker/ICD failure and initiate appropriate therapy. The patient's electrophysiologist/cardiologist can also be an invaluable resource in these cases and should be contacted early during the emergency department evaluation.
Multimedia
 | Media file 1: Intermittent periods of ventricular capture. |
 | Media file 2: Complete heart block. |
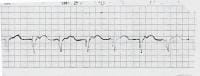 | Media file 3: 100% ventricular paced rhythm. |
pacemakers, defibrillator, internal defibrillator, automatic internal cardiac defibrillator, cardiac contraction, cardiac tachydysrhythmia, implantable cardioverter-defibrillators, ICD, ICDs, AICD, AICDs.
Source : http://emedicine.medscape.com/article/780825-overview?src=emed_whatnew_nl_0#ICDindication






